Changes to the regulation of all vapes in Australia.
Changes to the regulation of vapes are being implemented in stages from 1 January 2024. Vapes include vaping substances, vaping accessories and vaping devices.
Import controls on all vapes were introduced on 1 January 2024 and 1 March 2024, together with changes to the regulations for therapeutic vapes. Only vapes that have been notified to the TGA as meeting TGA requirements can be imported – other requirements also apply: see Information for importers.
From 1 July 2024, the Therapeutic Goods and Other Legislation Amendment (Vaping Reforms) Act 2024 (the Act) imposes new domestic controls on all vapes.
From 1 October 2024, therapeutic vapes containing nicotine or a zero-nicotine substance will be available for supply in pharmacy settings to patients 18 years or over without a prescription. Until 30 September 2024, patients need to speak with a medical or nurse practitioner to:
get a prescription to buy vapes containing nicotine, and
access zero-nicotine vapes
Further changes to the regulatory standards for therapeutic vapes are expected to commence later in the year, including to reduce permissible nicotine concentrations for all therapeutic vapes.
Cannabis vapes are subject to different requirements. For more information, see the Medicinal cannabis hub.
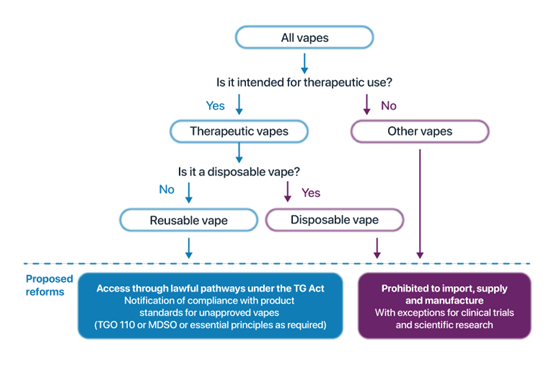
Regulation of vapes flow chart
The following chart outlines how vapes can be accessed:
Changes from 1 July 2024
Key changes from 1 July:
The domestic manufacture, commercial possession and sale of non-therapeutic vapes and disposable vapes is banned, regardless of whether they include nicotine or other controlled substances.
Vapes for smoking cessation, or the management of nicotine dependence, continue to be available from a pharmacy if they meet TGA regulatory requirements. However, patients need to speak with a medical or nurse practitioner to get a prescription to buy vapes containing nicotine, and access zero-nicotine vapes.
Introduction of a strengthened advertising framework for vapes. The framework bans advertising of vapes, except where specifically authorised. The ban covers all media platforms, including social media, as well as other forms of advertising, promotion and sponsorship.
New offence and civil penalties for unlawful importation, domestic manufacture, supply, advertisement and commercial possession of vapes.
Organisations responsible for enforcement now have greater powers to investigate non-compliance, seize unlawful goods and share relevant information with other compliance and enforcement bodies, both across the Commonwealth as well as states and territories.
Flavours of therapeutic vapes have been restricted to mint, menthol and tobacco. Transitional arrangements that allowed other flavours to be supplied will end.
Requirements for vapes at 1 July 2024
To be lawfully imported and supplied in Australia, therapeutic vapes must comply with the regulatory requirements in the Therapeutic Goods Act 1989. As unapproved therapeutic goods, at a minimum, vapes must meet the following product standards:
for vaping substances: Therapeutic Goods (Standard for Therapeutic Vaping Goods) (TGO 110) Order 2021 (TGO 110)
for vaping devices or accessories, either:
the Essential Principles or
the Therapeutic Goods (Medical Device Standard—Therapeutic Vaping Devices) Order 2023 (MDSO). The MDSO only applies to vaping devices and accessories that were excluded from the therapeutic goods regulatory framework prior to 1 January 2024.
Under TGO 110:
flavours are restricted to mint, menthol and tobacco
the maximum nicotine concentration limit is 100 mg/mL (base form or equivalent base form concentration).
Patients cannot lawfully access vapes that do not comply with these requirements.
For further information on product standards, please visit: Information for sponsors, importers and manufacturers.
Business surrender scheme and other transitional arrangements
The Therapeutic Goods (Vaping Goods – Possession and Supply) Determination 2024 (the Determination) provides exceptions for businesses and individuals to possess and supply vaping goods in certain circumstances without being in breach of relevant offences or civil penalty provisions.
Under the Determination, additional time is available for the:
surrender of unlawful vaping goods through the TGA business surrender scheme (a minimum quantity applies)
transport, storage or disposal of vapes
export of unlawful vapes, and
other limited activities.
These transitional arrangements will operate for a limited period and require certain actions to be taken and strict conditions to be met to qualify for the exception. Where a person is relying upon an exception in the Determination for their possession or supply of vaping goods, credible evidence must be produced upon request to demonstrate compliance with the relevant requirements and conditions. Where evidence is not produced, the goods and associated activity will be treated as unlawful and enforcement action may be taken.
More information:
Business surrender scheme for vaping goods
Possession and supply of vaping goods in Australia: Guidance on the legal instrument: Therapeutic Goods (Vaping Goods—Possession and Supply) Determination 2024
Changes from 1 October 2024
From 1 October 2024, therapeutic vapes with a nicotine concentration of 20mg/mL or less will be available from Australian pharmacies to patients 18 years or over without a prescription where a pharmacist assesses this to be clinically appropriate.
Therapeutic vapes for patients under 18 years and/or with a nicotine concentration of more than 20 mg/mL will continue to be available with a prescription from a medical or nurse practitioner, subject to state and territory laws. The laws in some jurisdictions prevent the supply of any vape to a person under 18.
Further information on the current regulation of vapes
Follow the links below to learn about the current regulations relating to the following groups:
Patients / consumers
Prescribers (medical and nurse practitioners)
Pharmacists
Importers, manufacturers and wholesalers
Retailers
Regulations and instruments
The vaping reforms are being implemented through the:
Therapeutic Goods Act 1989- external site and instruments made under it
Customs (Prohibited Imports) Regulations 1956- external site and instruments made under it.
The relevant instruments are:
Therapeutic Goods Regulations 1990- external site- external site
Customs (Prohibited Imports) Regulations 1956- external site- external site
Therapeutic Goods (Medical Devices) Regulations 2002- external site- external site
Therapeutic Goods (Excluded Goods) Determination 2018- external site- external site
Therapeutic Goods (Medical Devices—Specified Articles) Instrument 2020- external site- external site
Therapeutic Goods (Articles that are Not Medical Devices) Declaration 2023- external site- external site
Therapeutic Goods (Medicines and OTG—Authorised Supply) Rules 2022- external site- external site
Therapeutic Goods (Medical Devices—Authorised Supply) Rules 2022- external site- external site
Therapeutic Goods (Biologicals—Authorised Supply) Rules 2022- external site- external site
Therapeutic Goods (Standard for Therapeutic Vaping Goods) (TGO 110) Order 2021- external site- external site
Therapeutic Goods (Exempt Monographs) Determination 2021- external site- external site
Customs (Prohibited Imports) (Vaping Goods) Approval 2023- external site- external site
Therapeutic Goods Legislation Amendment (Vaping Reforms) Regulations 2024- external site- external site
Therapeutic Goods (Vaping Goods—Possession and Supply) Determination 2024- external site
Therapeutic Goods (Determined Goods) Determination 2024- external site- external site
Therapeutic Goods (Information Specification—Therapeutic Vaping Goods and Vaping Goods) Instrument 2024- external site- external site
Therapeutic Goods (Vaping Goods—Advertising) Authorisation 2024- external site- external site
Therapeutic Goods (Authorised Officers) Authorisation (No. 2) 2024
Therapeutic Goods (Authorised Persons) Authorisation (No. 2) 2024
Therapeutic Goods (Medical Devices—Authorised Persons) Authorisation (No. 2) 2024
Therapeutic Goods (Excluded Goods) Amendment (Vaping) Determination 2024- external site
Therapeutic Goods (Vaping Goods) Determination 2024- external site
Therapeutic Goods (Vaping Goods—Other Indications) Determination 2024- external site
Stay updated
Information will continue to be published on the TGA website. Please check back regularly for updates.
Related links
New regulations to place stronger controls on importation, manufacture and supply of vapes
Legislation
https://www.tga.gov.au/products/unapproved-therapeutic-goods/vaping-hub/changes-regulation-vapes
电子雾化与HNB产品都是新型电子产品,结构虽小,却融合应用多种材料、表面处理、芯片电子等技术工艺,而且雾化技术一直在不断更迭,供应链在逐步完善,为了促进供应链企业间有一个良好的对接交流,艾邦搭建产业微信群交流平台,欢迎加入;Vape e-cigarettes (VAPE) and Heat-Not-Burn e-cigarettes (HNB) are both emerging electronic products. Despite their compact size, they integrate various materials, surface treatment technologies, chip electronics, and other advanced technical processes. Moreover, atomization technology is constantly evolving and the supply chain is being progressively perfected. To facilitate good communication and networking among supply chain enterprises, Aibang has established an industry WeChat group communication platform and warmly welcomes interested enterprises to join.

